Proanthocyanidins as an Antioxidant
There are three main mechanisms for antioxidant activities of proanthocyanidins. They are free radical scavenging, inhibition of pro-oxidative enzymes, and chelation of transition metals.
Free radical scavenging
The basic concept of free radical scavenging activity of an antioxidant is a redox transition, involving donation of a single electron to a free radical. In this process, the radical character is transferred to the antioxidant, yielding an antioxidant-derived radical.
To act as an antioxidant, a free radical scavenger should produce a more stable and therefore less harmful compound after reacting with a free radical.
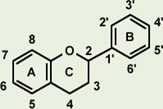
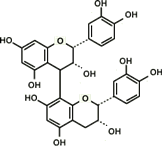
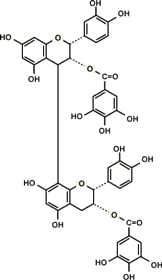
Proanthocyanidin-derived radicals are highly stable partly because a stabilizing hydrogen bond is formed between the radical in the B ring and an OH group in another B ring (Fig. 8). Polymerization of up to trimers increases free radical scavenging capabilities and the capabilities start to decline with further polymerization. The highest scavenging activity of all proanthocyanidins tested was found in the galloylated proanthocyanidins. This is produced when a gallic acid is introduced at the position 3 of proanthocyanidins, thereby significantly increasing the number of OH functional groups (Fig. 9). This form of proanthocyanidins is identified in the grape seed extract but not in pine bark extract.
In an in vitro comparative study, proanthocyanidins were 3 to 4 times more effective than Vitamin C and 50 to 75 % more effective than Vitamin E in the activities of radical scavenging and protection from DNA damage and lipid peroxidation.
Inhibition of enzymes
Proanthocyanidins can also exibit antioxidant activity through inhibition of pro-oxidative enzymes. These enzymes are either oxygenases that incorporate a molecular oxygen into its substrate, or oxidases that oxidize its substrate by removing electrons, transferring them to a molecular oxygen. These redox reactions are often catalyzed by transition metal cofactors.
With molecular oxygens directly participating, oxygen metabolizing reactions are inherently pro-oxidant. This is because any leaked electron is avidly picked up by a readily available oxygen molecule, whose electron affinity is second only to fluoride, resulting in an oxygen molecule with an unpaired electron (Superoxide radical).
Lipoxygenases and xanthin oxidase are two oxygen metabolizing enzymes that have been shown to be inhibited by proanthocyanidins. Lipoxygenases are involved in the reactions that generate leukotriens (see II under "Anti-inflammatory activities" below). Xanthin oxidase catalyses degradation of purine nucleotides to uric acid.
When xanthin oxidase is inhibited, the final products of purine metabolism are xanthin and hypoxanthin, which are more water-soluble than uric acid and less likely to form crystalline deposits as seen in gout.
Proanthocyanidins may inhibit both lipoxygenases and xanthin oxidase by chelating iron that is an essential cofactor of these enzymes.
Chelation of transition metals
Transition metals, such as iron and copper, are essential cofactors of several enzymes (oxygenases and oxidases) that are involved in oxygen metabolism. These metals are usually harmless because they are bound to proteins such as transferrin and ferritin for iron and ceruloplasmin for copper. However, when they are present in a free state in biological systems, they can catalyze free radical reactions.
These reactions are supposed to be involved in asbestosis, because asbestos contains high concentrations of iron. In hereditary hemochromatosis, excessive intestinal absorption of iron results in free iron (hemosiderin) deposits in tissues, such as the heart, liver and pancreas, causing failure of these important organs.
Agents that complex and harness these metals, therefore, dramatically decrease the deleterious biological effects of free radicals. For the chelating capability of proanthocyanidins, the catechol function of the B ring is very important and the chelating capacity of proanthocyanidins increases with the degree of polymerization.
Copyright 2006 Kuma.us